
A global specialty Pharmaceutical company selected POWERS to improve efficiencies to get its products to market more rapidly. After an in-depth analysis, our team helped them overcome significant production challenges and find time they didn’t know they had.

Project Overview
Performance Results
Situation
A global specialty Pharmaceutical company that develops, manufactures and markets safe, innovative and cost-effective generic pharmaceutical products selected POWERS to help them improve efficiencies. The company offers specialty dosage and sterile injectable drug products and is advancing an R&D pipeline of potential new products.
Project Objective
The time required to move products from bulk completion to product release had grown to a sluggish average of 40 days with a $3.1 million back order. POWERS was engaged to improve release cycle time and release efficiencies.
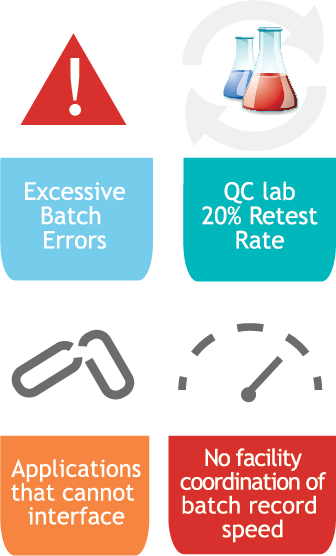
Challenges

Our Strategy
The POWERS team implemented robust QC Lab reporting tools to monitor efficiency, test quality and test cycle times. A Supply Chain Operating report was implemented to tie together production, QC Lab and QA performance metrics.
We created a batch record cycle time database, improving visibility and transparency of the approval and sign-off process.
Our team also developed a QC Lab Staffing Tool, improving staffing efficiencies.
Global Specialty Pharmaceutical Company
A global specialty Pharmaceutical company that develops, manufactures and markets safe, innovative and cost-effective generic pharmaceutical products selected POWERS to help them improve efficiencies. The pharmaceutical company offers specialty dosage and sterile injectable drug products and is advancing an R&D pipeline of potential new products.
Problems
- Excessive batch errors
- QC lab 20% retest rate
- Applications that cannot interface
- No facility coordination of batch record speed
Objectives
The time required to move products from bulk completion to product release had grown to a sluggish average of 40 days with a $3.1 million back order. The Powers Company was engaged to improve release cycle time and release efficiencies.
Strategies
The POWERS Company implemented robust QC Lab reporting tools to monitor efficiency, test quality and test cycle times. A supply Chain Operating report was implemented to tie together production, QC Lab and QA performance metrics.
Created a batch record cycle time database, improving visibility and transparency of the approval and sign-off process.
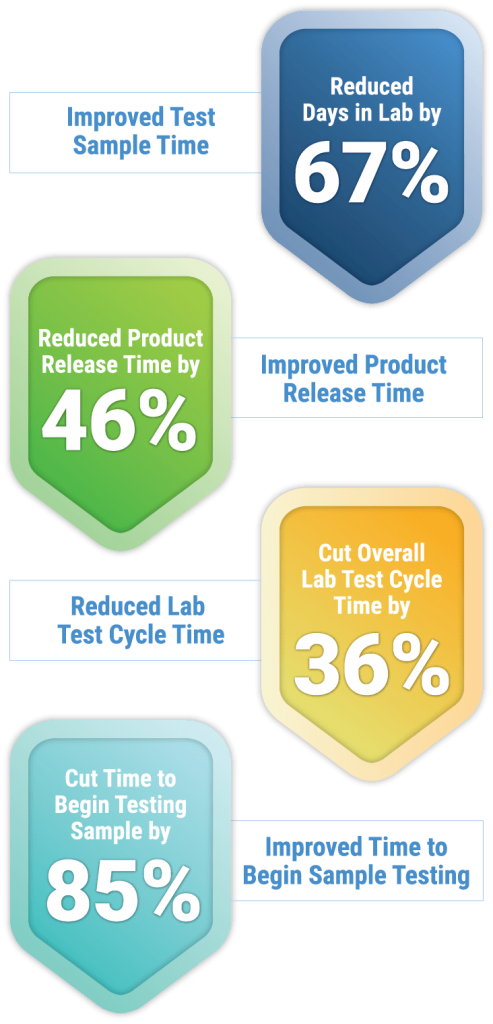
Results
67%
Reduced days in lab test sample time
46%
Reduced product release time
36%
Reduced overall lab test cycle time
85%
Reduced time to begin testing samples





